 |
Fig.1 Charge curve and structure change of O3 type NaNi0.5Mn0.2Ti0.3O2 cathode material
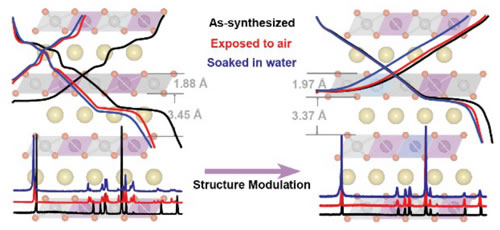
Fig.2 Structure and electrochemical performance of O3 type NaNi0.45Cu0.05Mn0.4Ti0.1O2 cathode material prepared by Cu/Ti co-doping
Due to the widespread distribution of sodium resources and low sodium salt costs, sodium-ion batteries are expected to be used in the large-scale energy storage field in the future. In recent years, researchers at the Key Laboratory of Molecular Nanostructures and Nanotechnology at the Institute of Chemistry, Chinese Academy of Sciences have conducted systematic explorations in search for positive electrode materials that can reversibly dissociate sodium ions. In previous studies, we developed zero strain characteristics (J. Mater. Chem. A 2015, 3, 4799), high sodium content (Nano Res. 2015, 8, 117) and high crystalline quality (Energy Environ. Sci. 2014, 7, 1643) A series of Prussian blue cathode materials for sodium ion batteries; and by constructing a three-dimensional conductive network structure, Prussian blue/carbon nanotube composite cathode materials with excellent low temperature performance (still work at -25°C) are obtained (Adv Mater. 2016, 28, 7243).
Recently, under the support of the National Natural Science Foundation of China, the Ministry of Science and Technology, and the Chinese Academy of Sciences, researcher Guo Yuguo, a researcher of the Institute of Molecular Nanostructure and Nanotechnology, has made new progress in the research of high performance layered oxide cathode materials. . Based on the theory of material dynamics calculations combined with experiments, they proposed the idea of ​​breaking the limitation of the single metal ion positive electrode, combining the advantages of various metal ions, to obtain O3-NaFe0.45Co0.5Mg0.05O2 cathode material with excellent overall performance (Adv. Energy Mater. 2017, doi: 10.1002/aenm.201700189).
In order to further promote the practical application of sodium-ion batteries, the researchers chose to conduct systematic research on nickel-manganese-based binary cathode materials with broad application prospects in the future. At present, this type of positive electrode material is divided into two types according to the content of sodium ion battery and the way of occupying the crystal structure: P2 type in which sodium ions occupy trigonal prism sites and O3 type in which sodium ions occupy octahedral sites. P2 type nickel-manganese-based layered cathode material will undergo irreversible P2-O2 phase transition in the high voltage region when the battery is working, which causes structural collapse of the material in the battery cycle, which limits its further application. In response to this problem, the Institute of Chemical Researchers found that the inactivation of P2-O2 phase of P2-Na0.67Mn0.67Ni0.33O2 cathode material can be effectively inhibited by inactive Mg doping, and the magnesium was revealed by cooperation with researchers of the Institute of Physics, Chinese Academy of Sciences. The charge/discharge process of the doped material is a single-phase reaction process, thereby improving the structural stability of the P2 type electrode material during charge and discharge, and significantly improving its cycle performance (Angew. Chem. Int. Ed. 2016, 55, 7445). ).
The sodium-rich O3 cathode material can meet the needs of low-cost sodium ion batteries in the future because of its high specific capacity, simple preparation method, and low raw material cost. However, these materials are faced with complicated phase transitions during charging and discharging, and air stability. The problem of poor sex (J. Mater. Chem. A 2016, 4, 17660). In view of this, the research team conducted in-depth systematic research on O3-type nickel-manganese-based binary cathode materials with high specific capacity, and cooperated with researchers from the Institute of Physics of the Chinese Academy of Sciences and the Brookhaven National Laboratory in the United States to combine real-time monitoring of in-situ X-rays. Diffraction, X-ray absorption spectroscopy, and atomic-scale SEM spectroscopy find that partial substitution of Mn4+ by Ti4+ can effectively suppress multiple phase transitions (>3V) in the high voltage region of O3-NaNi0.5Mn0.5O2 positive electrode (Fig. 1). Indicated), while increasing the diffusion coefficient of bulk sodium ions, greatly improved the material's long cycle performance and rate performance under high current density, related research results were published in the recent Adv. Mater. (2017, doi:10.1002/adma. 201700210).
In view of the poor air stability of O3-type layered cathode materials that spontaneously shift into the sodium-depleted phase in the air, the researchers proposed a structural optimization strategy for Cu/Ti co-doping (Figure 2). Through rationally modulating the composition and structure of NaNi0.5Mn0.5O2 material, and combining with density functional theory calculation simulation and material structure-electrochemical experiments, the O3 type NaNi0.45Cu0.05Mn0 obtained by Cu/Ti co-doping technology is proved. .4Ti0.1O2 cathode material can successfully inhibit the spontaneous release of sodium ions and the oxidation of the material, significantly improve the structural stability and capacity retention of the material exposed to air or even soaked in water, reduce the material storage costs, and effectively promote Its practical application also provides scientific guidance for the future design of cathode materials for high-performance sodium ion batteries and material structure optimization (J. Am. Chem. Soc. 2017, 139, 8440).
Blue Tungsten Oxide,Tungsten Oxide,Remelted Tungsten Trioxide,Sintered Tungsten Trioxide
HUNAN ZHONGNAN ANTIMONY&TUNGSTEN TRADING CO.,LTD , https://www.znat.com.cn
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)